
Since 2010, the Global Law Experts annual awards have been celebrating excellence, innovation and performance across the legal communities from around the world.
posted 3 months ago
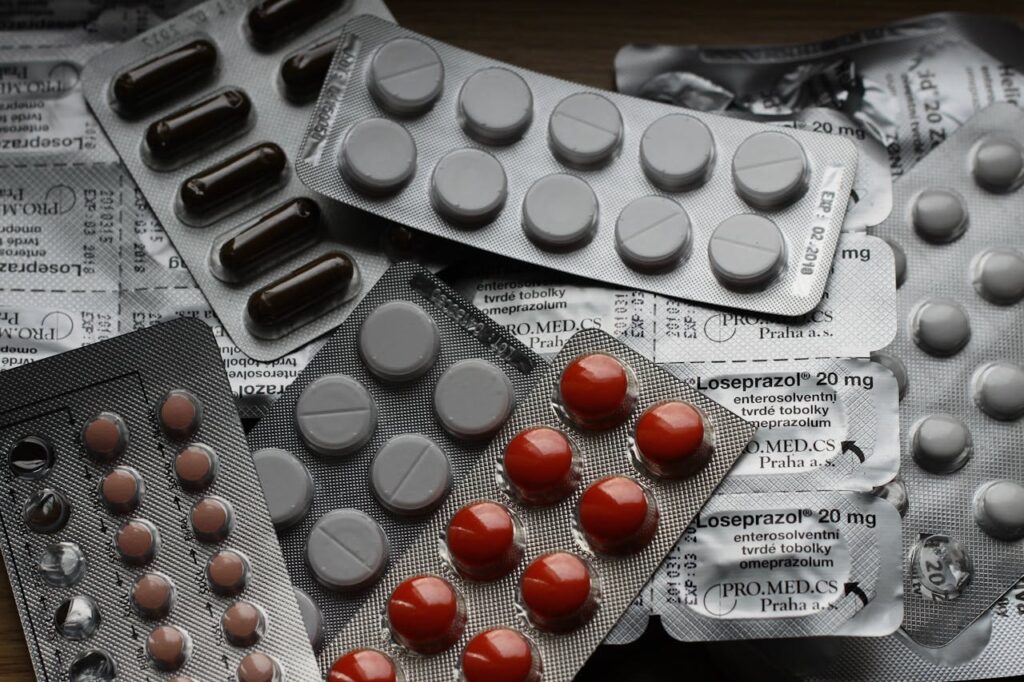
Thailand’s Food and Drug Administration (FDA) has proposed draft amendment to the Announcement on Criteria, Procedures, and Conditions for Applying, Issuing, and Renewing Certificates of Good Manufacturing Practice (GMP) for Pharmaceutical Products from Foreign Manufacturing Facilities B.E. …. (3rd amendment) (“Draft Announcement”). After a public hearing period ending on 1 May 2024, the Draft Announcement will be presented to the Minister of the Ministry of Public Health for consideration and approval before being published in the Royal Gazette.
The proposed revisions aim to update and simplify the requirements outlined in the previous 2nd Issue of the announcement, which has been enforced since 24 March 2023. The key goals are to reduce administrative burdens for drug importers in preparing application documents, while also enabling a more streamlined and efficient evaluation process by FDA officials. This will allow more effective regulatory oversight of imported drug product quality based on current industry practices.
Key proposed changes to the certification criteria include:
The Draft Announcement allows for a more flexible, risk-based approach to verifying current good manufacturing practices at foreign facilities based on available documentation from trusted regulatory authorities. Highly regulated facilities under PIC/S would have streamlined certification renewals, while foreign sites producing orphan drugs or veterinary medicines would follow requirements commensurate with their risk profiles.
Overall, these proposed changes aim to increase efficiency for both FDA regulators and the industry while maintaining strict oversight of drug product quality, safety, and efficacy standards for imported pharmaceutical products in Thailand. The streamlined certification framework reduces administrative burdens while focusing inspection resources based on risk.
If approved, the Draft Announcement will provide an updated regulatory system better adapted to current pharmaceutical manufacturing practices and global regulatory collaboration on GMP inspections. Both importers and the Thai FDA could benefit from a more modern, agile system for ensuring public health protection.
Author


There are no results matching your search.
Resetposted 6 hours ago
posted 17 hours ago
posted 17 hours ago
posted 4 days ago
posted 4 days ago
posted 4 days ago
posted 5 days ago
There are no results matching your search.
ResetSign up for the latest advisory briefings and news within Global Advisory Experts’ community, as well as a whole host of features, editorial and conference updates direct to your email inbox.
Naturally you can unsubscribe at any time.
Global Advisory Experts is dedicated to providing exceptional advisory services to clients around the world. With a vast network of highly skilled and experienced advisers, we are committed to delivering innovative and tailored solutions to meet the diverse needs of our clients in various jurisdictions.